- metadata:
- source: https://www.trialsitenews.com/a/pfizers-sensitive-document-reveals-alarming-facts-about-trial-subjects-f9d8876c
- people: [[Sonia Elijah]]
- related: [[2022-05-07 On what basis are pregnant women being encouraged to take the Pfizer vaccine]]
---
# Pfizer’s ‘Sensitive’ Document Reveals Alarming Facts about Trial Subjects
---
This is the latest installment of my ongoing [investigation](https://www.trialsitenews.com/p/sonia_elijah) into the court-ordered release of Pfizer’s trove of [documents](https://phmpt.org/pfizers-documents/), which the FDA relied on to grant emergency use authorisation for the Pfizer-BioNTech COVID-19 vaccine, back in December 2020. A particular document that stood out to me in the latest July 1, Pfizer data dump, is the 3611-page document entitled, ‘[C4591001-fa-interim-narrative-sensitive](https://pdata0916.s3.us-east-2.amazonaws.com/pdocs/070122/125742_S1_M5_5351_c4591001-fa-interim-narrative-sensitive.pdf).’
It contains pertinent information on hundreds of Pfizer’s clinical trial subjects who whether due to death, serious adverse events, pregnancy, COVID-19 or just ‘no longer meeting eligible criteria,’ were withdrawn from the trial. The document also includes revealing narrative comments for subjects in both the placebo group and those administered either a single or two-dose BNT162b2 (Pfizer-BioNTech COVID-19 vaccine) during Pfizer’s pivotal C4591001 trial, conducted in multiple sites spanning, South Africa, Argentina and the US. The bulk of the 3000+ page document pertains to the placebo group; however, this investigative report focuses on the narrative comments for the vaccine recipients.
### The Deaths of Subjects #10071101, #11621327 and #11521497
The screenshot below reveals how subject #10071101 succumbed to a fatal cardiac arrest. A relatively young 56-year-old female was given two doses of BNT162b2 on the following dates: 30 July and 20 August 2020.
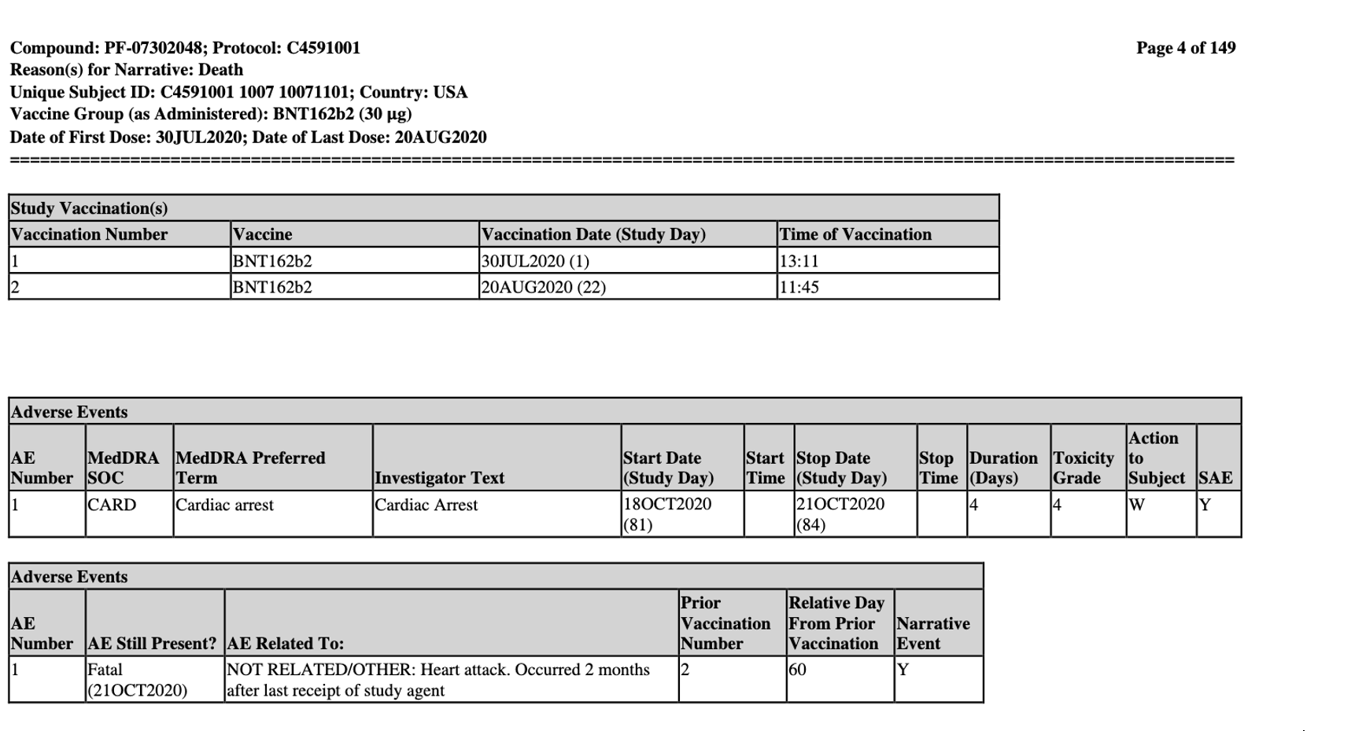
The narrative comment reads _‘In the opinion of the investigator, there was no reasonable possibility that the cardiac arrest was related to the study intervention of clinical trial procedures, as the death occurred 2 months after receiving Dose 2. Pfizer concurred with the investigator’s causality assessment.’_
The conclusion that ‘_there was no reasonable possibility’_ the vaccine could have caused the fatal cardiac arrest because ‘_death occurred 2 months after receiving Dose 2’_ is not only presumptuous but also lacks a robust medical assessment. This is evident by the further comment that ‘_it was unknown if an autopsy was performed_.’ Why was there no follow up or inquiry into whether an autopsy was performed?
Cardiac arrest is listed in the 8-page appendix of adverse events of special interest (AESIs) in Pfizer’s own cumulative analysis of post-authorisation adverse event reports, which I analysed back in December for [Trial Site News.](https://www.trialsitenews.com/a/fdas-forced-hand-drops-pfizers-bombshell-safety-document)
Turning to the death of subject #11621327, a 60-year-old male was given his first and last dose of the vaccine on September 10, 2020, since he died three days later. (See screenshot below).
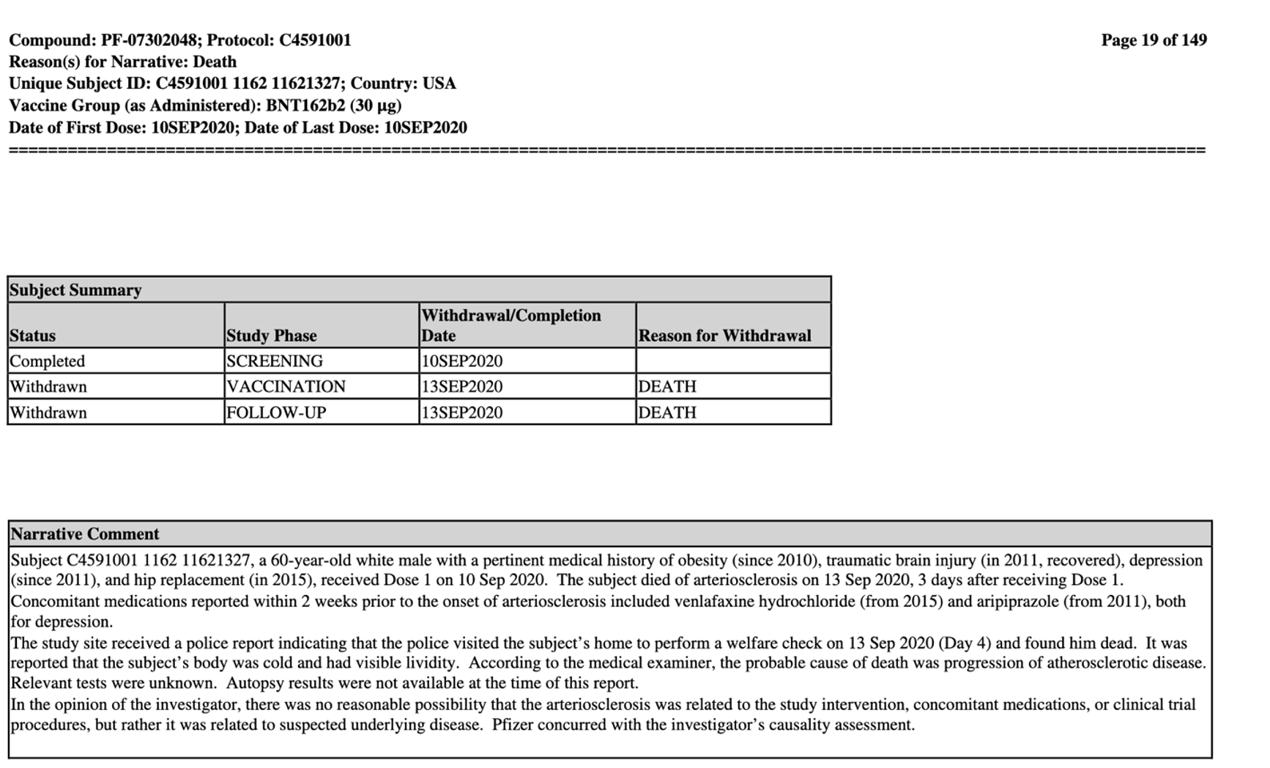
_‘Probable cause of death was progression of atherosclerotic disease’,_ however, in the subject’s medical history there is no mention of this condition, however, obesity is mentioned. Since death followed very shortly after the date of his first dose- could the vaccine have accelerated this disease? Or was it due to another cause, since ‘_autopsy results were not available at the time of this report_,’ these questions remain unanswered.
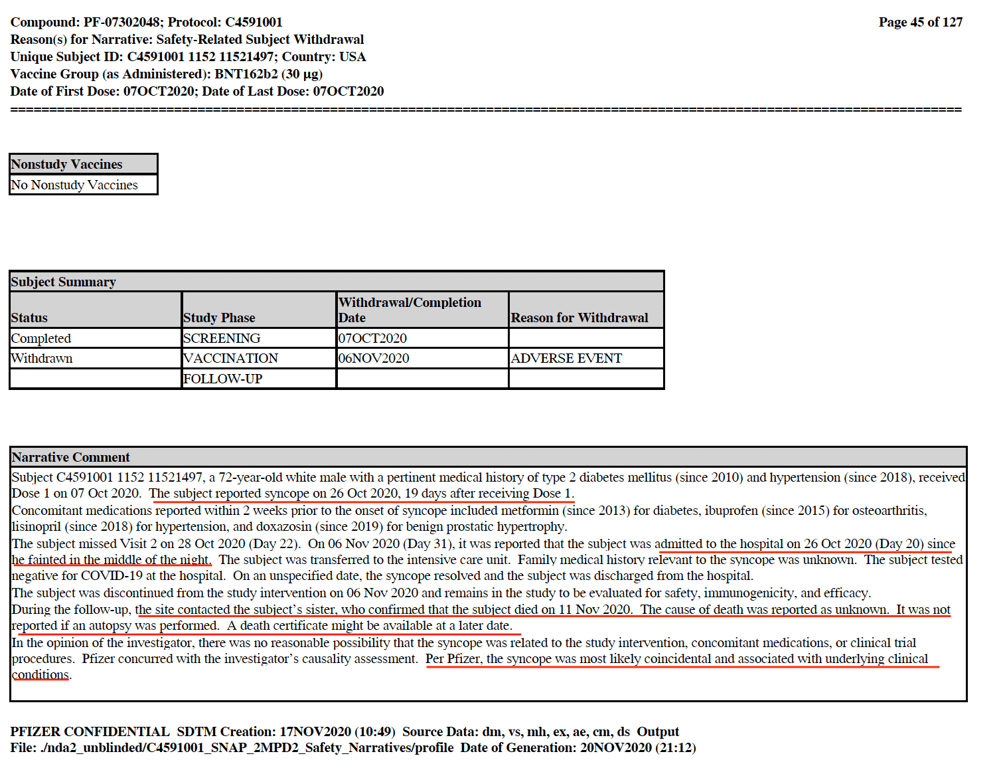
Another case that caught my attention was subject #11521497, a 72-year-old male who received the first dose of the vaccine on 7 October. On 26 October, he was admitted to hospital because ‘_he_ _fainted in the middle of the night’._ This serious adverse event was reported as a syncope (a temporary loss of consciousness usually related to insufficient blood flow to the brain). On 11 November the subject was reported to have died. The investigator opined there was _‘no reasonable possibility that the syncope was related to the study intervention’_ and Pfizer believed the syncope was ‘_most likely coincidental_.’ What’s unusual is that ‘_cause of death is reported as unknown’_ and neither the trial investigator nor Pfizer even attempt to investigate. (See screenshot below)
### The Related Serious Adverse Events
What’s interesting is that in every case where a SAE (serious adverse event) was possibly related to the vaccine in the trial investigator’s opinion, Pfizer ‘**_did not concur_**_._’ (See screenshot below).
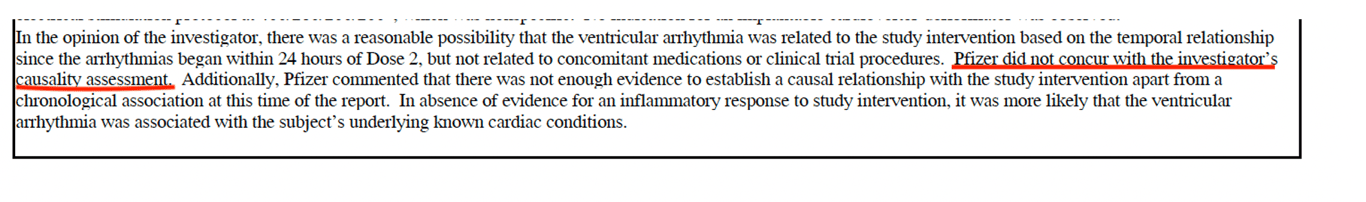
The screenshot above shows the narrative comments for Subject #11421247, a 71-year-old female, who developed severe ventricular arrhythmias, the very same evening she received the second dose of the vaccine, on 14 October. According to the trial investigator ‘_there was reasonable possibility that the ventricular arrhythmia was related to the study intervention \[vaccine\]_.' However, according to Pfizer there was _‘not enough evidence to establish causal relationship.’_ Arrhythmia is another AESI listed (among 8 pages) in Pfizer’s own cumulative analysis of post-authorisation adverse event reports.
A prime example of Pfizer’s dismissive conduct in failing to consider the possibility that its vaccine could be related with a subject’s serious adverse event, is the case of subject #11781107. The 48-year-old female developed lymphadenopathy with ‘_at least 4 enlarged lymph nodes’_ after receiving her first and which ended up being her last dose of the vaccine. The narrative comment (see screenshot below) is quite shocking. It reveals that even after the hospital oncologist believed the vaccine to be the ‘_most likely etiology \[cause\] for her lymphadenopathy’_ and the trial investigator opined, ‘_there was a reasonable possibility that the lymphadenopathy was related to the study intervention, **Pfizer did not concur**_..’
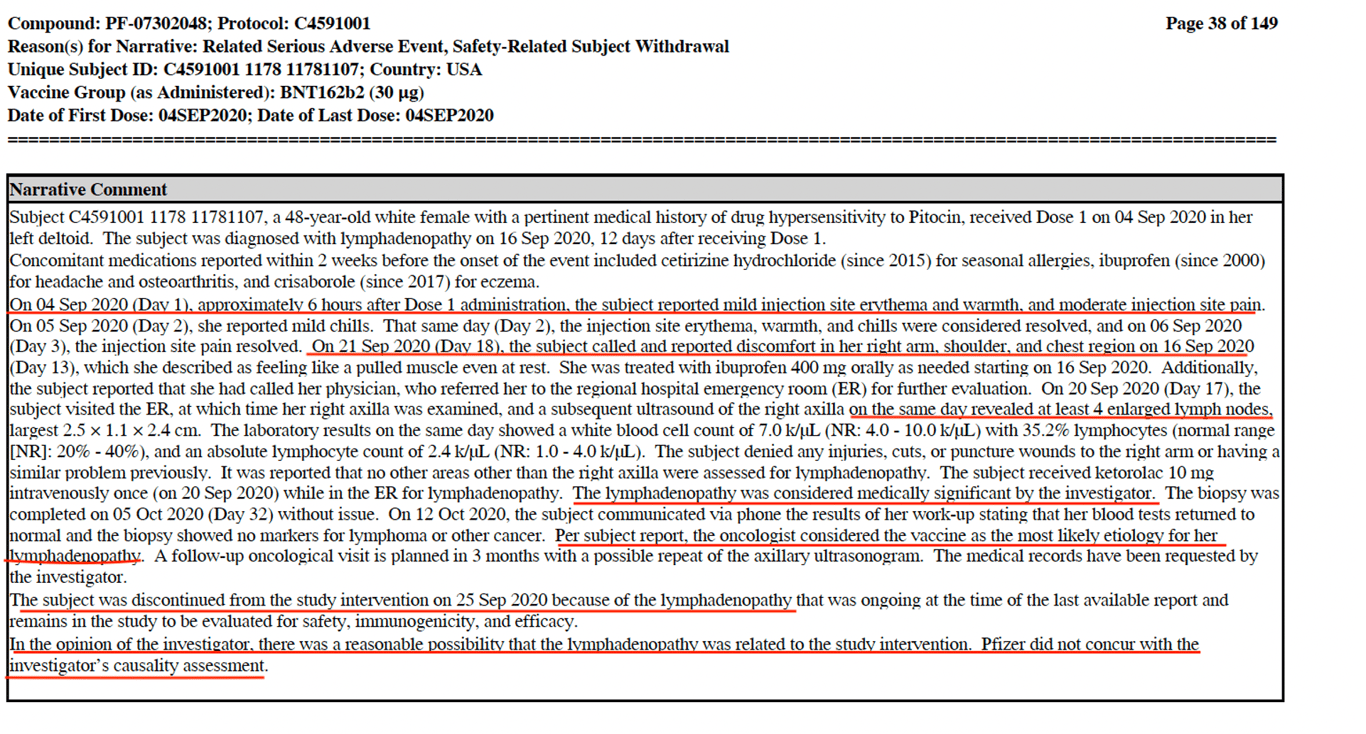
A study published in _[Cell](https://www.cell.com/cell/pdf/S0092-8674(22)00076-9.pdf)_ by Röltgen et al, showed that vaccinal spike protein and mRNA persisted in the human lymph nodes up to 8 weeks post vaccination. Furthermore, in Pfizer’s own non-clinical overview released by court order on [March 1](https://phmpt.org/wp-content/uploads/2022/03/125742_S1_M2_24_nonclinical-overview.pdf), the toxicity studies conducted on rats showed ‘_increased size of draining iliac lymph nodes_.’ Also, in my recent [interview](https://rumble.com/v1bev6n-david-wiseman-discusses-fda-meeting-to-authorize-eua-covid-vaccine-for-chil.html) with David Wiseman a video clip was shown of the June 14-15 VRBPAC meeting, which revealed the alarming scene of Pfizer’s senior Vice President of Vaccine Clinical R & D, Dr William Gruber completely avoiding a series of critical scientific questions asked by Dr Portnoy, on how much spike protein is produced by the cells and for how long it persists? Given Gruber's evasive response, one can confidently assume that this study along with many other known safety studies (as reported in another of my investigative [reports](https://www.trialsitenews.com/a/first-look-at-newly-released-pfizer-docs-part-2) for Trial Site News) were never done.
### The Pregnant Women in the Trial
I came across several subjects who had positive pregnancy tests post-first-dose. These women were withdrawn from the study, according to Pfizer’s own clinical protocol. In these cases, towards the end of the narrative comment section it stated: _‘The subject was discontinued from the study intervention…because of the exposure during pregnancy and remains in the study to be evaluated for safety, immunogenicity, and efficacy.’_ Given whistle-blower Brook Jackson’s revelations of fraudulent activities in the way Ventavia (a subcontractor of Pfizer) ran its trial sites, putting patient safety at risk- it begs the question were these pregnancies ever followed up?
One pregnancy outcome which was known for certain, was for subject #12411279. The subject received the first dose on 19 August and reported an _‘exposure during pregnancy on 10 September 22 after receiving Dose 1.’_ What is alarming is that on 5 October she ‘_experienced vaginal haemorrhage’_ then on 14 October she _‘presented to the emergency room..and had a spontaneous abortion \[miscarriage\].’_ (See screenshot below).
_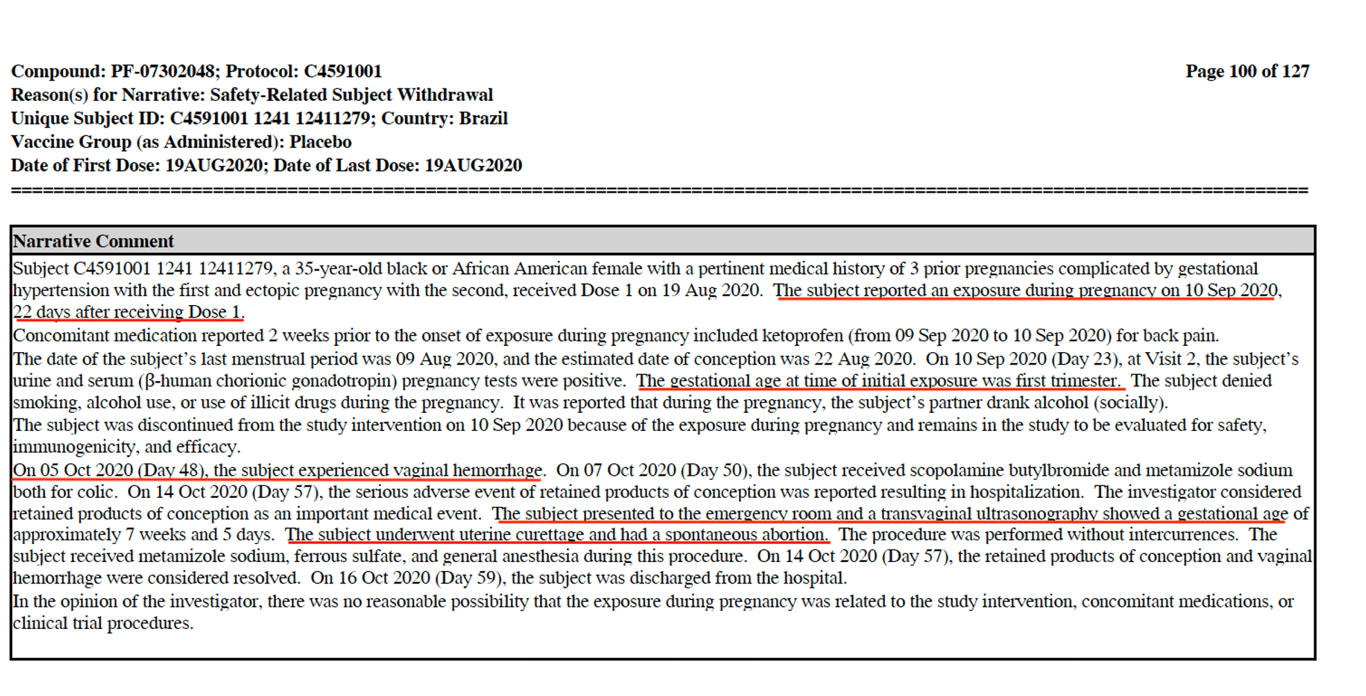_
What is astounding is that there is no mention in the narrative comment section, whether the vaccine may have caused the miscarriage. I’ve written a [report](https://www.trialsitenews.com/a/on-what-basis-are-pregnant-women-being-encouraged-to-take-the-pfizer-vaccine-6e3730c1) for Trial Site News pertaining to the Pfizer vaccine and it being recommended for pregnant woman by the CDC and other health agencies around the world. Shockingly documented in one of Pfizer’s own reports is the fact that out of 270 pregnancies, 23 were reported as spontaneous abortions and for a staggering 238 pregnancies no outcomes were ever reported.